environmental benefit of wastewater treatment plants in mountainous areas in the alps

|
Comparison of technology, costs and environmental benefit of wastewater treatment plants in mountainous areas in the alps |

|
Keywords
Problem description
Methods
Results and discussion
Conclusions
Acknowledgement
Experimental units
Small wwtp, seasonal operation, carbonate, settling, nitrification, alkalinity
During a 4 years monitoring program the performance of 12 small cyclic activated sludge systems at mountain refuges (110 to 260 pe) have been investigated. The following operational problems have been identified which might be of general interest for activated sludge on-site treatment plants loaded with high concentrated wastewater:
- In treatment plants with seasonal operation and significant load variations it is difficult to obtain a constant optimum sludge concentration. The changing F/M ratio and the poor maintenance can cause a varying sludge volume index.
- Nitrification of high ammonia concentrations in wastewater from tourist resorts and sites with low sanitary standards causes a lack of alkalinity within the system.
- As a further consequence a low pH value causes decreasing nitrification rates and negative effects on the sludge settling properties due to an increased fraction of fine particles.
In order to investigate the outlined problems and to develop countermeasures two parallel lab-scale SBR systems have been operated under defined boundary conditions. Both reactors have been made from transparent vertical pipes with 192 mm diameter and 2.1 m height and a volume of 60 l each. One SBR operation cycle was divided into an 2 hours aeration phase (fine bubbles aeration with constant air flow) and an anoxic settling phase of 1 hours. Twice a day 5 litres of municipal wastewater enriched with beer and ammonia-chloride was added (2950 mg COD/l, 10 mg Norganic/l, 158 mg NH4-N/l). To improve the situation 30 g marble powder (0.5 g CaCO3/l) has been added into one of the reactors and COD, nitrogen-, carbon-fractions, pH, DO, temperature and sludge parameters have been monitored.
Starting with inoculated sludge from a municipal wwtp (sludge volume index SVI of about 60 ml/g) the added artificial wastewater with relatively low suspended solids concentration generally tends to deteriorate the sludge settling properties. An addition of 0.5 g CaCO3/l led immediately to an partial solution (0.2 g/l calcite was ionised to Ca+2, CO3-2, HCO3- and CO2) while the concentration of fixed suspended solids (residue from sludge ignition) was increased by 0.3 g/l. By sludge sampling at 4 locations vertically distributed over the height of each pipe the calcite concentration profiles during settling have been detected. The vertical profiles of fixed suspended solids are clearly correlated with the concentration of total suspended solids. This fact indicates the close incorporation of calcite into the activated sludge. The portion of calcite contributes to the weight of the sludge flocs until calcite is completely dissolved according to the carbonic acid balance. This process is represented by the development of the sludge volume index in pipe A after calcite addition (Fig.1).
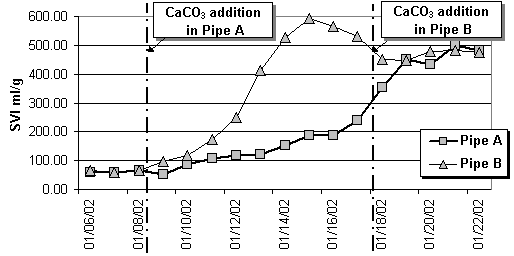
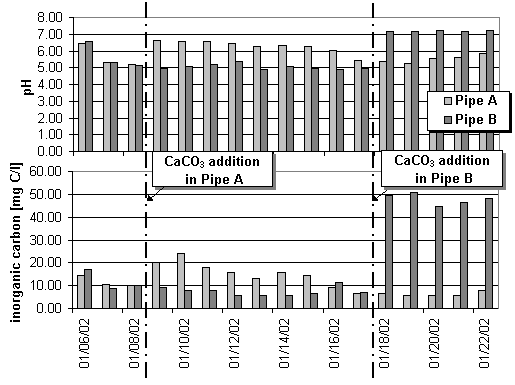
As a consequence from carbonate addition two direct effects could be measured: Partly solution of calcite is indicated by the increased inorganic carbon concentration in the filtered samples and the fixation of H+ cations during the establishment of the inorganic carbon equilibrium is indicated by a significant pH-increase (Fig.2). Bicarbonate is known as the carbon source for nitrifying organisms. Nitrification of high ammonia concentrations leads to an acidification (pH is around 5 in Fig.2). The low pH-level tightens the substrate limitation of nitrifying organisms because most of the bicarbonate is transfered to carbon dioxide or carbonic acid respectively. Therefore the carbonate addition improves the situation with regard to both effects – increase of the inorganic carbon concentration and of the pH-level (and consequently of the bicarbonate fraction).
In order to evaluate the effects on the nitrification rate nitrogen balances of both SBR-systems have been drawn. The nitrogen content of the influent flow, the effluent flow, the waste sludge withdrawal and the nitrogen accumulation in the system (organic nitrogen in the activated sludge increased from 10.1 g to 13.8 g Norganic/g SS) was considered. The average nitrification rate after calcite addition in pipe A was 0.86 mg N/l.h and dropped to 0.58 mg N/l.h while in pipe B after addition the rate increased from 0.30 to 0.98 mg N/l.h (Fig.3).
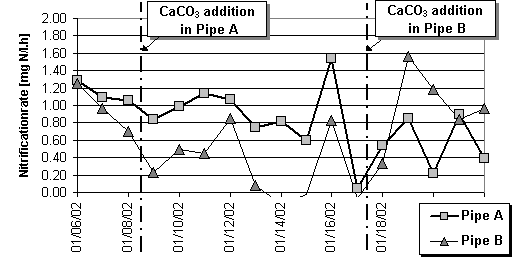
Results from lab-scale experiments indicate that carbonate addition significantly improves activated sludge settling properties and nitrification efficiency from ammonia concentrated wastewater. As a major advantage in comparison with liquid alkaline precipitants, calcite is bounded to the sludge and not washed out with the liquid phase. It is slowly consumed according to the alkalinity demand from nitrification. Especially for seasonal on-site treatment plants a sufficient carbonate depot added together with sludge inoculation promises a reliable sludge development during the following weeks. On-site experiments are currently conducted.
The results of this presentation are achieved within the project „Comparison of technology, costs and environmental benefit of wastewater treatment plants in mountainous areas in the Alps“, which is supported by the LIFE-program of the European Commission.

Photo 1: Filtration of samples taken after a settling period of 45 min (CaCO3 in Pipe A)

Photo 2: The difference in the sludge color during aeration (CaCO3 in Pipe B)

Photo 3: The difference in the sludge structure during settling (CaCO3 in Pipe B)

Photo 4: The pipes after settling (CaCO3 in Pipe B)